Pptx 4236 KB. John Hayes Created Date.

How To Find The Natural Abundance Of An Isotope
7 Calculate the relative molecular mass of each of the following compounds.

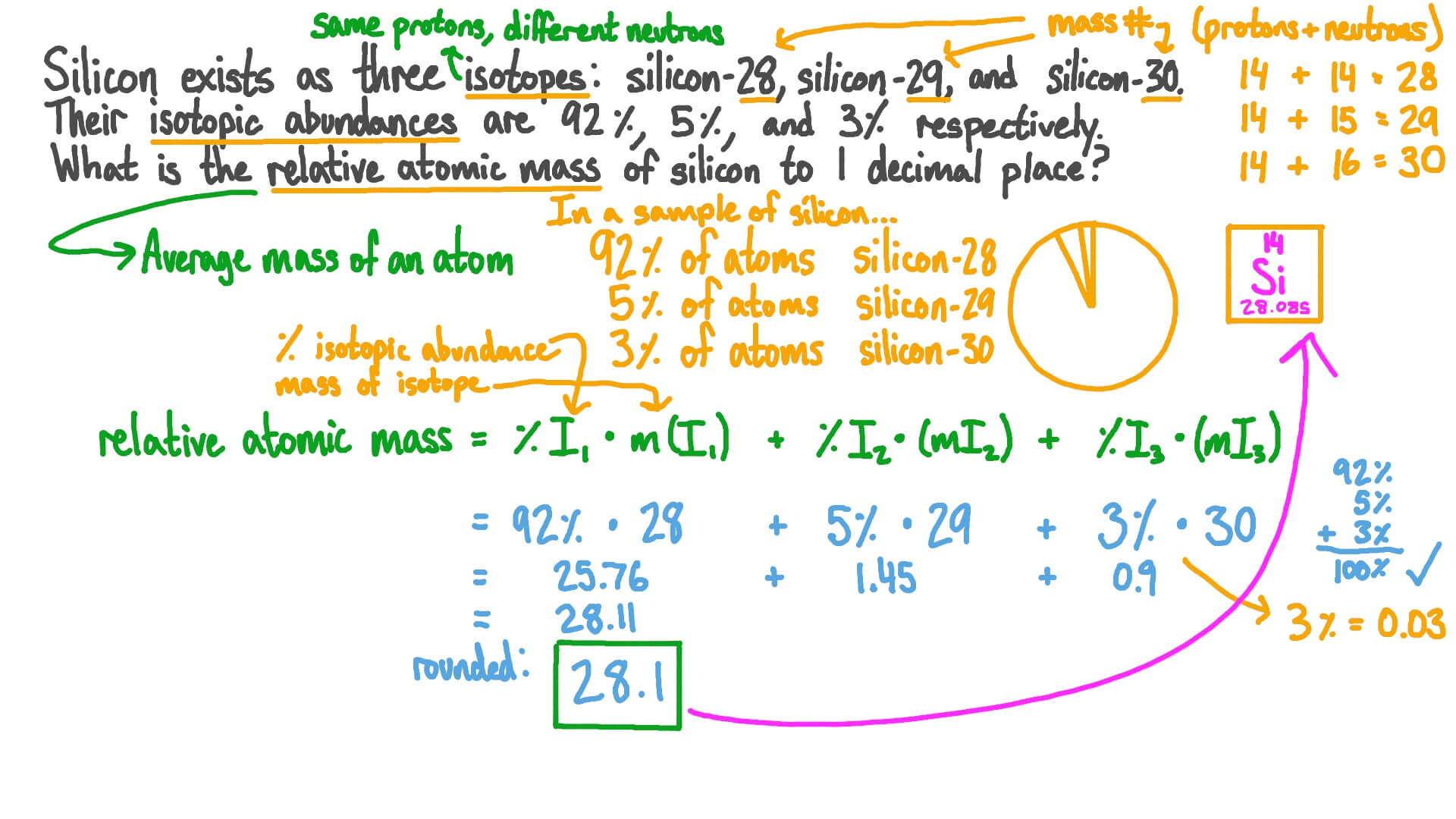
. Isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. If you set the equation as a decimal this means the abundance would be equal to 1. Calculate the average atomic mass of strontium.
Note that this equation is limited to two isotopes. Using the following data calculate the average atomic mass of magnesium give your answer to the nearest 01 u. Calculate the average atomic mass of chlorine.
Calculate the relative atomic mass of strontium. How To Calculate Percent Abundance Isotope. For example the average abundance of 12C is 9889 while the average abundance for 13C is 111.
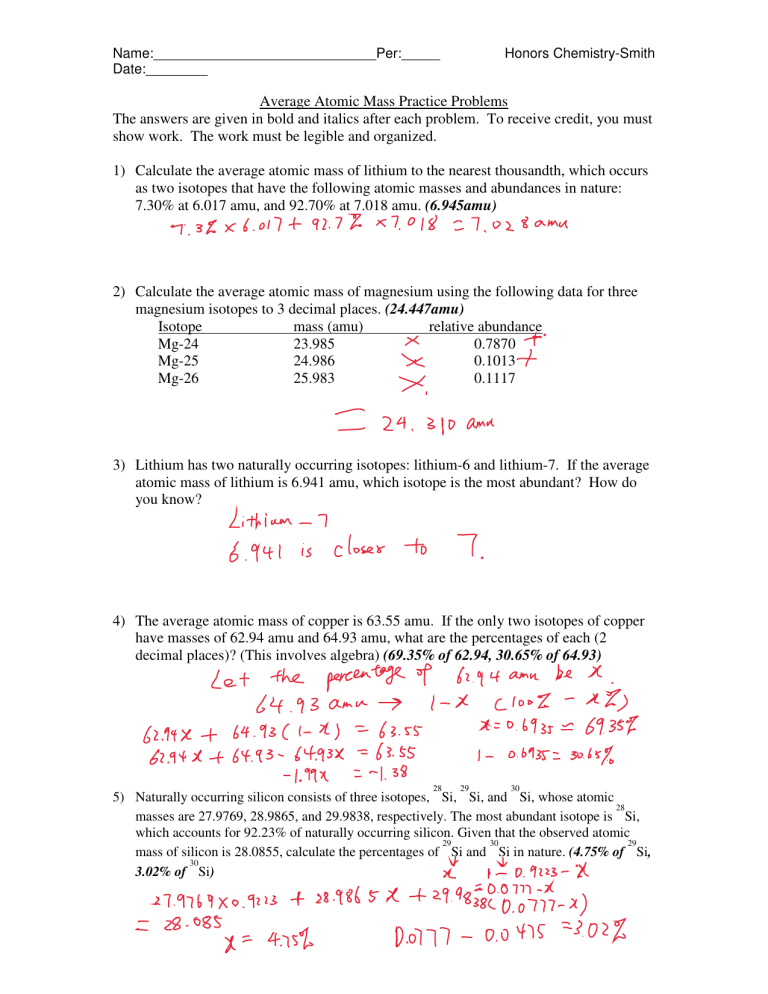
Calculate the actual atomic mass of 65Cu. Using the periodic table. Copper-63 which has an atomic mass of 6293 u and copper-65 which has an atomic mass of 6493 u.
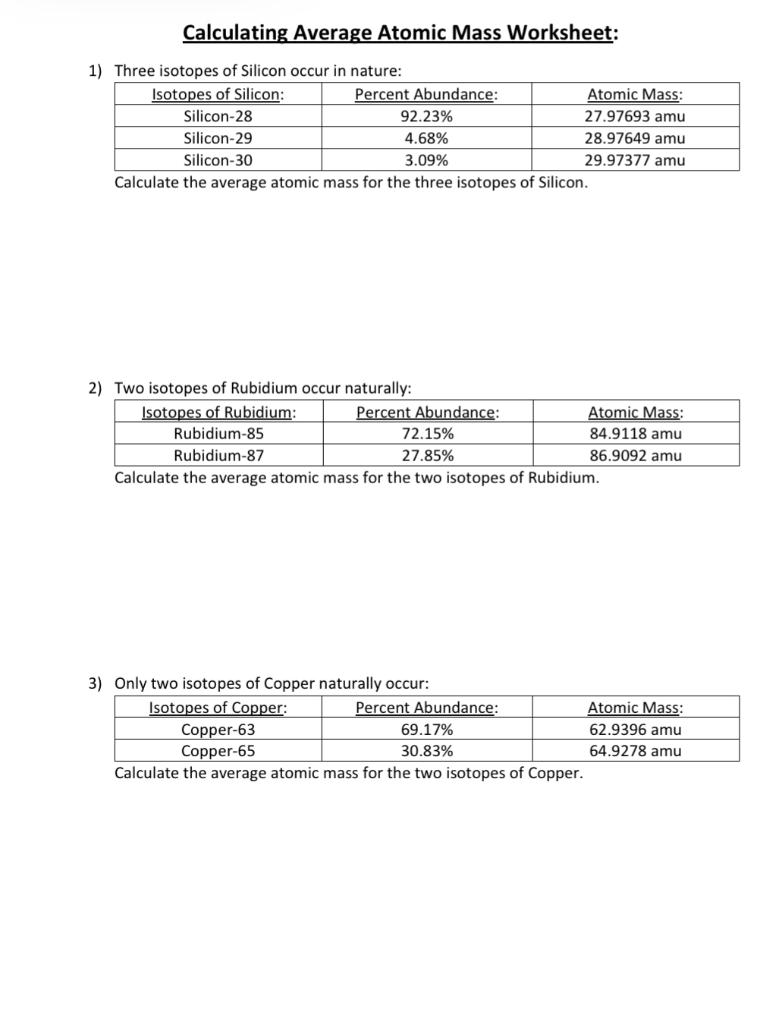
Naturally occurring boron B consists of two isotopes with a mass of 10 and 11. The element bromine has three isotopes naturally appealing. Calculating Average Atomic Mass Worksheet Answers.
The percent abundance of these isotopes is as follows. The average atomic mass between these two isotopes is 63546 amu. Using the following data calculate the average atomic mass of magnesium give your answer to the nearest 01 u.
12 25Mg Percent abundance. Worksheet to help students calculate RAM from relative abundances of isotopes. For example about the source of the carbon and about processes in atom percent 13C 13C12C C100 1 which the carbon has participated.
I find this approach helps students to understand the principle of a weighted average. A 2797693 x 9223 100 2897649 x 468 100 2997377 x 309 100 25803. Chlorine consists of two isotopes with masses of 35 abundance 75 and 37 abundance of 25.
To calculate the percent abundance of each isotope in a sample of an element chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100. Europium-151 has an abundance of 4803 and Europium-153 has an abundance of 5197. A closely related term is the fractional abundance The delta notation.
Calculate the atomic mass of strontium. The three isotopes of lead are shown below each with its percent by mass abundance and the composition of its nucleus. Strontium consists of four isotopes with masses of 84 abundance 050 86 abundance of 99 87 abundance of 70 and 88 abundance of 826.
Carbon is composed primarily of two isotopes. The equation would then become. Determine both the percentage abundance and the relative isotopic mass of the second isotope.
Avg Mass mass mass. The table below outlines the average isotopic abundances of elements that are most commonly measured for stable isotope measurements. Ã 79 U 81 mg Calculates the elementary atomic.
Average atomic massthe weighted average of the masses relative abundance of all the isotopes of an element. 46Ti 80 47Ti 78 48Ti 734 Ti 55 49 50Ti 53. Usually one isotope is the predominantly abundant isotope.
Natural Isotopic Abundances of Light Stable Isotopes. 79Br of I r 7892 and percentage abundance 5054 and 81Br. 12 26 Mg Percent abundance.
Average mass of an element atomic mass of isotope I X percent abundance of isotope I100 atomic mass of isotope II X percent abundance of isotope II100. Relative abundance of isotopes worksheet learning objectives Define atomic weight Calculate atomic weight from percent abundance Manipulate the atomic. Chlorine has two stable isotopes 3 5 C l and 3 7 C l with atomic masses 349689 u and 369659 u respectively.
Calculate the average atomic mass of bromine showing all work. 65Cu 649278 amu 7 Magnesium consists of three naturally occurring isotopes. Microsoft Word - IsoCalcs30Sept04doc Author.
Last updated October 9 2019. Strontium consists of four isotopes with masses of 84 abundance 050 86 abundance of 99 87 abundance of 70 and 88 abundance of 826. Calculating percent abundance of isotopes worksheet Calculating percent abundance of isotopes worksheet pdf.
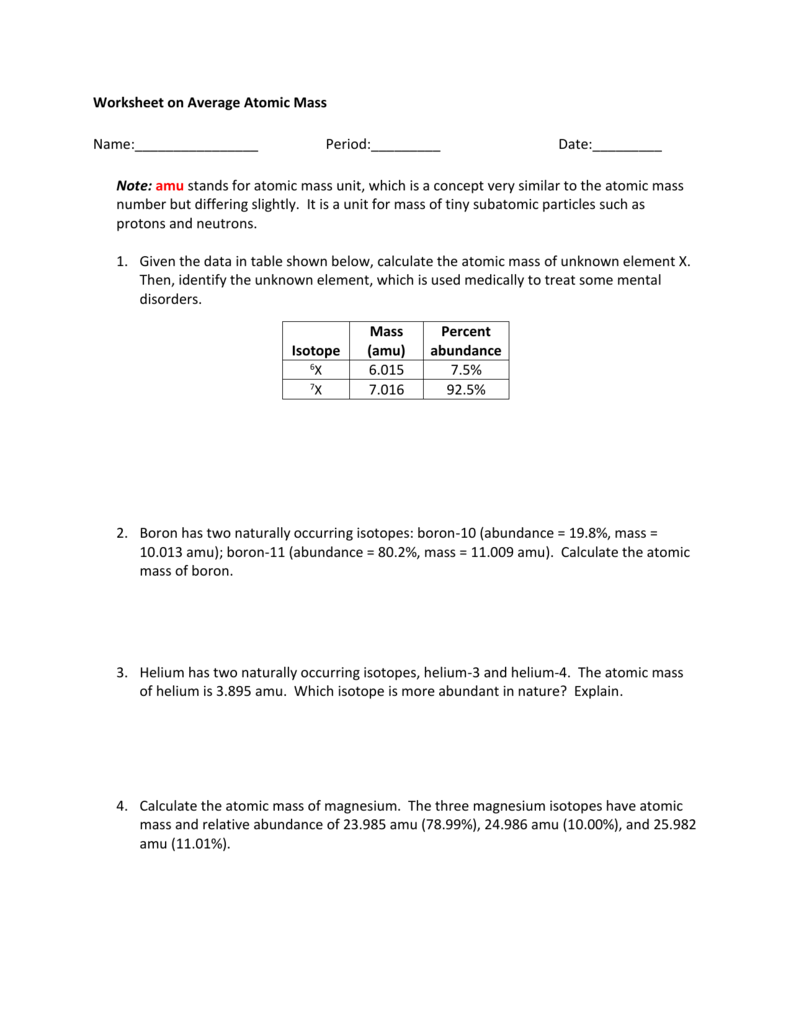
For example the atomic mass of carbon is reported as 12011 amu atomic mass units. Boron-10 has an abundance of 187 and boron-11 has an abundance of 813. And pb 208 207 98 amu.
This problem demonstrates finding the percent abundance of isotopes with known average atomic mass. SIMULATION in Isotopes Atomic Mass Subatomic Particles. The equation would then become.
24Mg 7870 25Mg 1013 and 26Mg 117. It has two isotopes. Absolute abundances of isotopes are com-differences as small as 000001 can provide information monly reported in terms of atom percent.
Titanium has five common isotopes. In the abundance of isotopes this second law is used to explain why potassium gets less dense when you heat it up. In any sample of.
If you set the equation as a decimal this means the abundance would be equal to 1. 12 25 Mg Percent abundance. Exact Weight Isotopic Mass Percentage Abundance.
Copper has two isotopes. Structured to start by thinking of balls in a box through to simple calculation for copper. In this worksheet we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses.
12 24Mg Percent abundance. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance. What is the average atomic mass of titanium.
12 26Mg Percent abundance. X 100-x 100 where the 100 designates the total percent in nature. Calculating percent abundance of isotopes worksheet.
The other isotope 65Cu has an abundance of 3091. 3 1 9 6. X 1 x 1.
The formula to find the percent abundance of an element with two isotopes is as follows. Use this information to determine what BR isotopes occurring in nature. As a percent the equation would be.
What is the relative atomic mass of Boron. Rubidium has two naturally occurring isotopes 85rb relative mass 84. What is the average atomic mass of europium.
12 24 Mg Percent abundance. Europium-151 has an abundance of 4803 and Europium-153 has an abundance of 5197. A Hydrogen perchlorate HOCl b Ammonium sulfate NH 4 2 SO 4 c Urea NH 2 2 CO d Hydrogen peroxide H 2 O 2 8 Calculate the relative.
A mass spectrum of molecular BR2 shows three peaks with mass numbers of 158 u 160 u and 162 u.
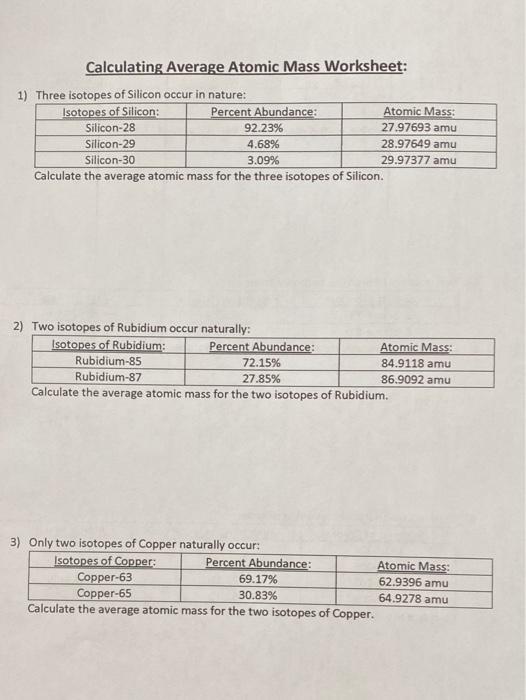
Solved Calculating Average Atomic Mass Worksheet 1 Three Chegg Com

How To Calculate Percentage Abundance Using Atomic And Isotopic Masses

Calculating Average Atomic Mass Worksheet Name Pdf Atomic Mass Unit Isotope
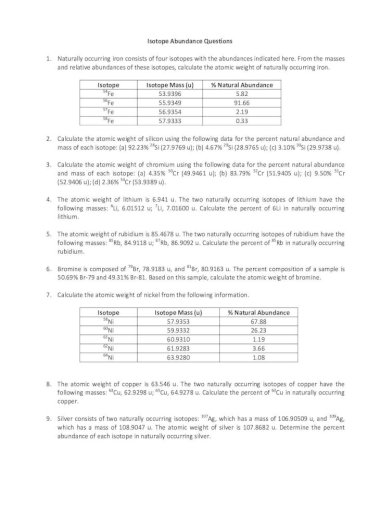
Isotope Abundance Worksheet Abundance Questions 1 Naturally Occurring Iron Consists Of Four Isotopes With The Abundances
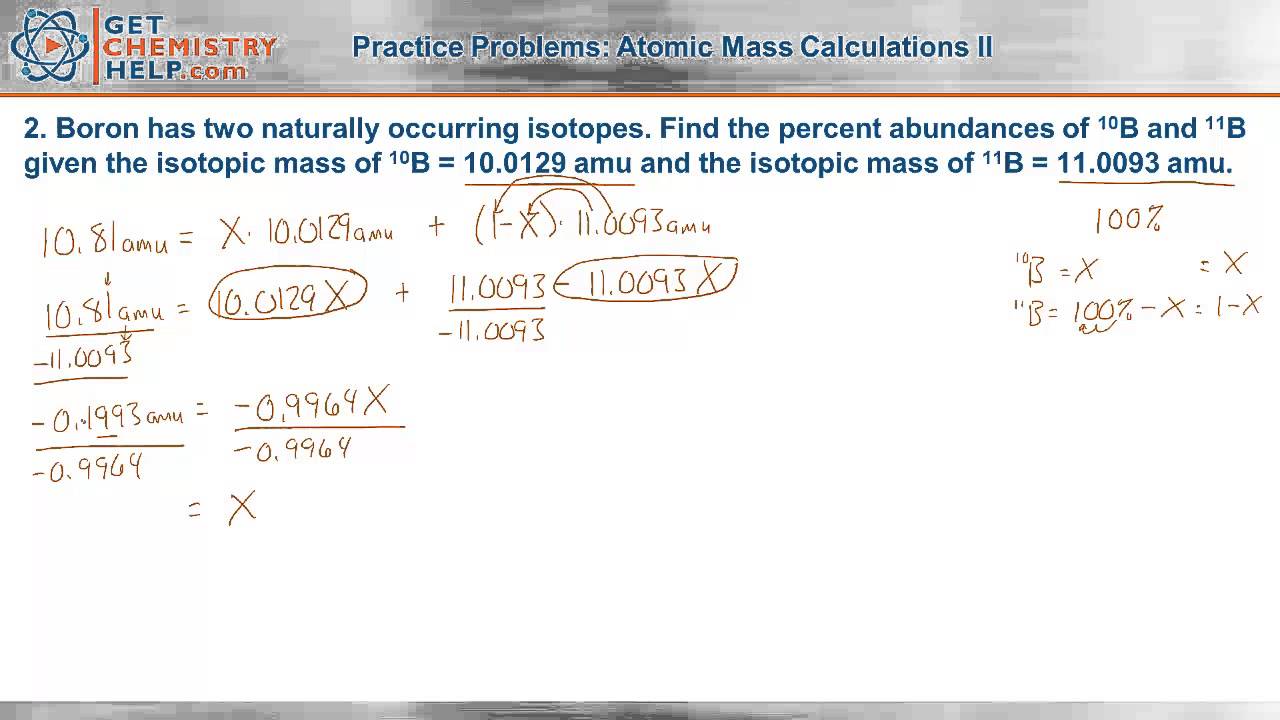
Chemistry Practice Problems Atomic Mass Calculations Ii Youtube

Isotopes Relative Atomic Mass Solutions Examples Videos

Average Atomic Mass And Percent Abundance Worksheet 2 And Key Pdf Pdf Isotope Chemical Elements

Solving For Percent Abundance With Isotopes Chemistry Sample Problem Youtube

Question Video Calculating The Relative Atomic Mass Of Chlorine From Isotopic Abundances Nagwa
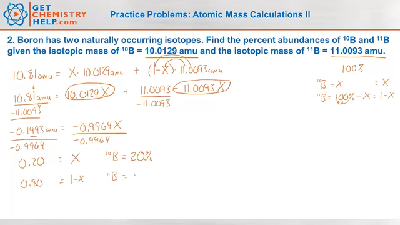
Chemistry Practice Problems Atomic Mass Calculations Ii Get Chemistry Help
Question Video Using Relative Abundance Of Isotopes To Calculate Relative Atomic Mass Nagwa

Handout 03 Average Atomic Mass

Isotopes Relative Abundance Calculations Structured Worksheet Teaching Resources

5 1 Isotopes And Average Atomic Mass Ppt Download
Solved Calculating Average Atomic Mass Worksheet 1 Three Chegg Com

Average Atomic Mass Worksheet Pdf

Year 11 Chemistry Relative Atomic Masses Mass Spectrometry Ppt Download
Tidak ada komentar:
Posting Komentar